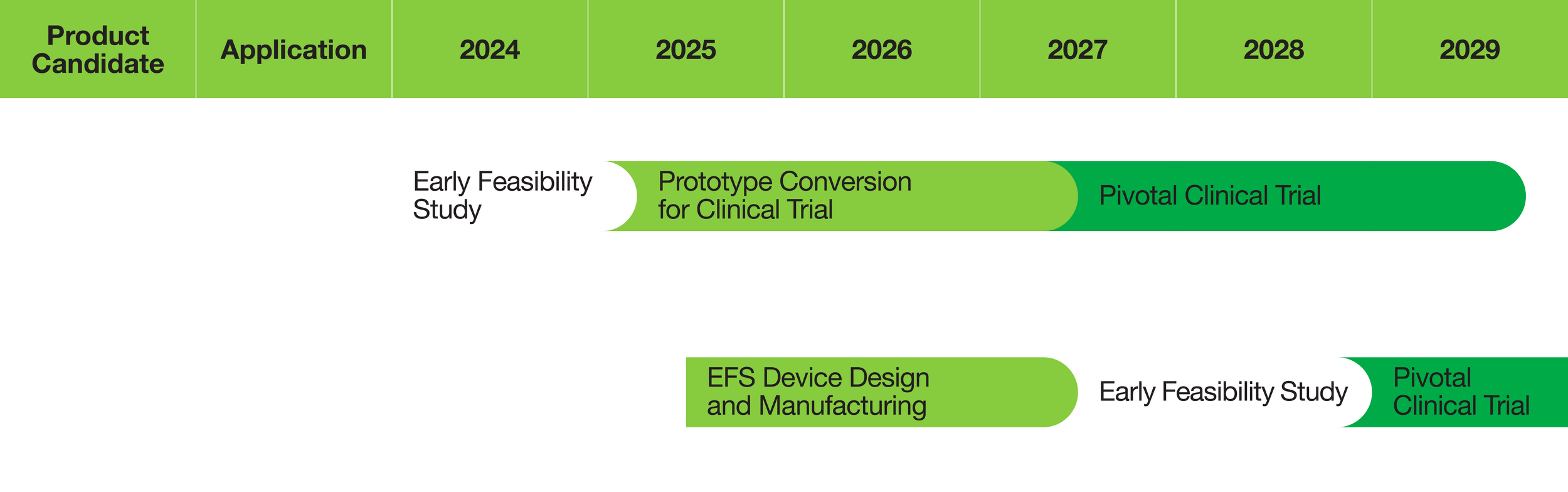
PIPELINE
Target clinical development timelines for our medical devices.
Our team is fully dedicated to executing our clinical development plan.
Argus II
Our legacy innovation for providing artificial vision.
Launched in 2011, The Argus® Retinal Prosthesis System (“Argus II”) is the world’s first FDA-approved² device for providing artificial vision to blind individuals with late-stage retinitis pigmentosa (RP).
Argus II has successfully helped hundreds of profoundly blind people see light and motion. Many of these patients have been using their Argus implants for over ten years, attesting to our high manufacturing standards and product reliability. NOTE: We discontinued sales of this product in 2019 to refocus our Argus II technology on developing Orion for potential use by a much broader population of blind people.
Orion®
An improved, next-generation device system undergoing clinical testing.
Building on the achievement of Argus II, we are conducting an Early Feasibility Study (EFS) to evaluate a more advanced system for artificial vision called The Orion® Visual Cortical Prosthesis System (“Orion”). This new system has the potential to make artificial vision available to a substantially larger group of profoundly blind individuals – including those affected by to glaucoma, diabetic retinopathy, eye trauma, optic nerve damage, and retinitis pigmentosa.
In the course of the EFS, safety and efficacy were evaluated at 36 months post-implantation. We are now planning a pivotal clinical trial based on these results.
Stroke Recovery System
Our newest product candidate for motion recovery after stroke.
Leveraging our neurostimulation technology, we are developing a new product candidate to improve muscle function in partially paralyzed stroke patients who are undergoing rehabilitation. The electrode array in this medical device system will be placed on the motor cortex (surface) of the brain to deliver neurostimulation.
Our goal is to improve hand and arm movement by applying stimulation to the motor cortex, thereby accelerating the recovery process and achieving a higher level of function.
[1] Estimated total addressable U.S. Market Size. Source: Company estimates.
[2] Authorized by Federal (U.S.) law under a Humanitarian Device Exemption to provide electrical stimulation of the retina to induce visual perception in blind patients with severe to profound retinitis pigmentosa and bare light or no light perception in both eyes. The effectiveness of this device for this use has not been demonstrated.